Bcs Class Iv
Posted : admin On 18.12.2018
Bcs Classification System
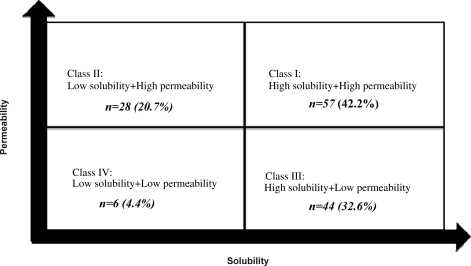
Bcs Classification
Biopharmaceutics classification system (BCS) class IV compounds, exhibits least oral bioavailability, low solubility and intestinal permeability among all pharmaceutical classes of drugs. Autocad free download 64 bit. Thus, these drugs need more compatible and efficient delivery system. • BCS class IV: “low” solubility – “low” permeability. Working document QAS/04.109/Rev.1 page 5 Depending on the classification, the oral availability of the API may be expected to range from heavily dependent on the formulation and manufacturing method (e.g. Class 2 APIs. Criteria may expand the number of Class 1 drugs eligible for a biowaiver of in vivo bioequivalence (BE) studies and provide predictability of drug disposition profiles for Classes 2, 3, and 4 compounds. KEYWORDS: BCS, BDDCS, Solubility, Permeability, Metabolism, Biowaiver INTRODUCTION BCS is the scientific framework for classifying drug. Introduction Overcoming the difficulties posed by BCS Class II and IV APIs, where oral bioavailability is typically poor, is a challenge for modern formulation.